Background: Ibrutinib (IBR) and venetoclax (VEN) combination is an effective therapy for patients (pts) with CLL. We previously reported results of the first-line cohort of a phase II trial of combined IBR and VEN for high-risk pts with CLL (Jain, NEJM 2019, JAMA Oncology 2021). Here we report updated data for 120 pts (80 pts in the original published cohort and 40 pts in an expansion cohort) with a median follow-up of 61.5 months.
Methods: Pts with previously untreated CLL meeting IWCLL treatment criteria were enrolled. All pts had at least one high-risk feature: del(17p), mutated TP53, del(11q), unmutated IGHV, or age ≥65 years. Pts received IBR 420 mg daily for 3 cycles followed by addition of VEN (weekly dose-escalation to 400mg daily). Combined therapy was given for 24 cycles (28 days/cycle). Pts with bone marrow (BM) undetectable MRD (U-MRD) (flow cytometry; sensitivity 10 -4) at 24 cycles of combined therapy discontinued both VEN and IBR; MRD+ pts continued IBR. A trial amendment allowed an additional 12 cycles of combined VEN and IBR for pts who remained BM MRD+ after Cycle 24. Response assessments included BM and CT studies (2008 IWCLL criteria). U-MRD was defined as <0.01%; low MRD+ 0.01% to <1%; high MRD+ ≥1%. Progression-free survival (PFS) was assessed as the time from the start of study drug to CLL progression, Richter transformation, or death from any cause. Blood MRD was monitored every 6 months after active therapy.
Results: Between August 2016 and February 2019, a total of 120 pts were enrolled. Median age was 64.5 years (range, 26-88 years). 86% had IGHV-unmutated CLL. 23% had del(17p)/ TP53 mutation. The median follow-up is 61.5 months.
Six pts came off study during 1 st 3 cycles of IBR monotherapy; 114 pts initiated VEN. After 12 cycles of the combination, 62/120 (52%) achieved BM U-MRD remission; 43/120 (36%) were BM MRD-positive (low MRD+, n=35; high MRD+, n=8). After 24 cycles of the combination, 77/120 (64%) achieved BM U-MRD remission; 24/120 (20%) were BM MRD+ (low MRD+, n=23; high MRD+, n=1). Overall, 86/120 (72%) achieved BM U-MRD as the best response. One pt had DLBCL transformation and 1 pt had CLL progression during the first 2 year of therapy (details below).
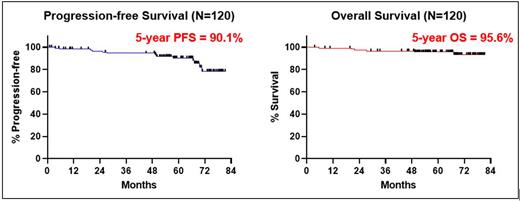
The 5-year PFS is 90.1% and 5-year OS is 95.6% (Figure 1). The 5-year PFS for pts with del(17p)/ TP53 mutation (n=27) is 86.1%.
Of the 77 pts who were BM U-MRD at the end of cycle 24 of the combination, 73 discontinued all therapy, 4 pts continued IBR per treating physician discretion. Among these 77 pts, with a median follow-up of 40 months post Cycle 24, 22 pts had recurrence of blood MRD (defined as MRD ≥0.01% in 2 consecutive visits). Of the 22 pts with MRD recurrence, 5 pt had CLL progression at a median of 18 months (range, 6-30 months) from MRD recurrence (details below); 16 are being monitored without any active therapy for CLL and without clinical disease progression; 1 pt died from mesothelioma. Among the 55 pts who remain in blood U-MRD remission, 53 remain in long-term follow-up in U-MRD remission; 1 pt had B-PLL transformation and 1 patient died (found unresponsive at home; was off therapy for 2 years).
There were 24 pts who were BM MRD+ at the end of cycle 24 of the combination (low MRD+, n=23; high MRD+, n=1). The only pt with high-MRD+ at end of cycle 24 was noted to have Richter transformation at that time. The remaining 23 pts (all low MRD+ in BM, range 0.01-0.95%) continued IBR monotherapy. With a trial amendment, MRD+ pts after Cycle 24 could get 12 additional cycles of VEN; 18/23 pts have resumed VEN. 11/18 (61%) pts achieved U-MRD remission during the third year of combined therapy. Only 2/23 pts are still receiving IBR (remaining pts have d/c IBR; all pts have d/c venetoclax); none of these 23 pts had clinical relapse.
A total of 6 pts had CLL progression (1 during the first 2 yr of therapy; remaining 5 during off-therapy phase). 3/6 had evaluation of BTK, PLGG2 and BCL2 mutations at the time of relapse and none were detected. 5 pts have started subsequent therapy (acalabrutinib, n=4; ibrutinib, n=1; all are clinically responding); 1 pt has not yet started therapy.
Conclusions: We report long term follow-up of combined IBR and VEN in first-line CLL (n=120) with a 5-year PFS of 90.1%. The 5-year PFS for pts with del(17p)/ TP53 mutation is 86.1%. Retreatment with BTK inhibitor appears effective for pts with disease relapse.
OffLabel Disclosure:
Jain:Mingsight: Research Funding; Medisix: Research Funding; Incyte: Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Ipsen: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Pfizer: Research Funding; Cellectis: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Beigene: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Loxo Oncology: Research Funding; Newave: Research Funding; TG Therapeutics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Genentech: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; BMS: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Aprea Therapeutics: Research Funding; Fate Therapeutics: Research Funding; MEI Pharma: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Precision Biosciences: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Takeda: Research Funding; Kite/Gilead: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Servier: Research Funding; ADC Therapeutics: Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; CareDX: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; AbbVie: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Novalgen: Research Funding; Dialectic Therapeutics: Research Funding; TransThera Sciences: Research Funding; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Pharmacyclics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding. Thompson:abbvie: Consultancy; adaptive biotechnologies: Consultancy, Research Funding, Speakers Bureau; astrazeneca: Consultancy, Speakers Bureau; beigene: Consultancy; genentech: Consultancy; janssen: Consultancy, Speakers Bureau; Lilly: Consultancy; merck: Consultancy, Speakers Bureau; pharmacyclics: Consultancy. Ferrajoli:Genetech: Honoraria; GenMab: Research Funding; Beigene: Research Funding; AstraZeneca: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Janssen: Honoraria. Senapati:Kite Pharma: Other: Advisory Board. Burger:AstraZeneca, Pharmacyclics: Other: Advisory Board, Research Funding; Janssen: Other: Speaker fees and Travel Support; Abbvie, Beigene: Research Funding. Borthakur:Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy; Pacylex, Novartis, Cytomx, Bio Ascend:: Membership on an entity's Board of Directors or advisory committees; Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding. Konopleva:AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Clinical Trials Support, Research Funding. Kadia:Pinotb-Bio: Consultancy; Astellas Pharma Global Development: Research Funding; Servier: Consultancy; Sanofi-Aventis: Consultancy; SELLAS Life Sciences Group: Research Funding; Regeneron Pharmaceuticals: Research Funding; Pulmotect, Inc.: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Consultancy; Liberum: Consultancy; Janssen Research and Development: Research Funding; Hikma Pharmaceuticals: Speakers Bureau; Iterion: Research Funding; GenFleet Therapeutics: Research Funding; Glycomimetics: Research Funding; Genentech: Consultancy, Research Funding; Delta-Fly Pharma, Inc.: Research Funding; Cyclacel: Research Funding; Cure: Speakers Bureau; Cellenkos Inc.: Research Funding; Celgene: Research Funding; AstraZeneca: Research Funding; Amgen, Inc.: Research Funding; Ascentage Pharma Group: Research Funding; Jazz Pharmaceuticals, Pfizer, Pulmotect, Inc, Regeneron Pharmaceuticals, SELLAS Life Sciences Group: Research Funding; Biologix, Cure, Hikma Pharmaceuticals: Speakers Bureau; Genzyme: Honoraria; AbbVie, Amgen, Inc, Ascentage Pharma Group, Astellas Pharma Global Development, Astex, AstraZeneca, BMS, Celgene, Cellenkos Inc, Cyclacel, Delta-Fly Pharma, Inc, Genentech, Inc., Genfleet, Glycomimetics, Iterion, Janssen Research and Development: Research Funding; Daiichi Sankyo, Genentech, Inc., Genzyme, Jazz Pharmaceuticals, Liberum, Novartis, Pfizer, PinotBio, Inc, Pulmotect, Inc, Sanofi-Aventis, Servier: Consultancy; Agios: Consultancy; BMS: Consultancy, Research Funding; Astex: Honoraria. Pemmaraju:Menarini Group: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Cimeio Therapeutics AG: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Neopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Magdalen Medical Publishing: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Medscape: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Curio Science: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSA Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ClearView Healthcare Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ImmunoGen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CareDx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pacylex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Harborside Press: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CancerNet: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Protagonist Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Stemline: Consultancy, Membership on an entity's Board of Directors or advisory committees; Intellisphere: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Imedex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dava Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Patient Power: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Aplastic Anemia & MDS International Foundation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; OncLive: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aptitude Health: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PeerView Institute for Medical Education: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Physician Education Resource (PER): Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASH Committee on Communications: Other: Leadership; ASCO Cancer.Net Editorial Board: Other: Leadership; Karger Publishers: Other: Licenses; United States Department of Defense (DOD): Research Funding; National Institute of Health/National Cancer Institute (NIH/NCI): Research Funding; HemOnc Times/Oncology Times: Other: Uncompensated; Bristol Myers Squibb Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Daver:Daiichi Sankyo: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Servier: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Hanmi: Research Funding; Trovagene: Research Funding; FATE: Research Funding; Novimmune: Research Funding; Glycomimetics: Research Funding; AROG: Consultancy; Novartis: Consultancy; Jazz: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Celgene: Consultancy; Kite, a Gilead company: Consultancy, Research Funding; Kronos Bio: Research Funding. Jabbour:Abbvie: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Ascentage Pharma Group: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Adaptive Biotech: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Hikma Pharmaceuticals: Consultancy, Honoraria, Research Funding. DiNardo:Schrödinger: Consultancy; Takeda: Honoraria; Fogham: Honoraria; Notable Labs: Honoraria; Novartis: Honoraria; BMS: Honoraria; ImmuniOnc: Honoraria; Servier: Honoraria; AbbVie/Genentech: Honoraria; Astellas: Honoraria. Alvarado Valero:BerGenBio: Research Funding; CytomX Therapeutics: Consultancy; Jazz: Research Funding; Astex: Research Funding; MEI Pharma: Research Funding; Sun Pharma: Consultancy, Research Funding; Daiichi-Sankyo: Research Funding; FibroGen: Research Funding. Yilmaz:Pfizer: Research Funding; Daiichi-Sankyo: Research Funding. Bose:GSK, Novartis, Karyopharm, AbbVie, Pharma Essentia, Jubilant, Morphic: Honoraria; Kartos, Telios, Ionis, Disc, Janssen, Geron: Research Funding; Incyte, BMS, CTI, Morphosys, Blueprint, Cogent, Sumitomo: Honoraria, Research Funding. Gandhi:Dava Oncology: Honoraria; Sunesis: Honoraria, Research Funding; AbbVie: Research Funding; LOXO: Research Funding; Clear Creek Bio: Consultancy, Research Funding; Pharmacyclics: Research Funding. Wierda:Accutar Biotechnology: Research Funding; Numab THerapeutics: Research Funding; Nurix THerapeutics: Research Funding; Juno Therapeutics: Research Funding; Janssens Biotech: Research Funding; Janssens Biotech Inc: Research Funding; GlaxoSmithKline: Research Funding; Loxo Oncology, Inc./Lilly: Research Funding; Cyclacel: Consultancy, Research Funding; Oncternal Therapeutics, Inc.: Research Funding; Miragen: Research Funding; Sunesis: Research Funding; KITE Pharma: Research Funding; Bristol Myers Squibb (Juno & Celgene): Consultancy, Research Funding; Gilead Sciences: Research Funding; AstraZeneca/Acerta Pharma: Consultancy, Research Funding; Pharmacyclics LLC: Research Funding; Genentech: Research Funding; AbbVie: Consultancy, Research Funding; GSK/Novartis: Research Funding; NIH P30 CA016672/MDACC Cancer Center Support Grant: Research Funding; National Comprehensive Cancer Network: Other: Nonrelevant Financial Relationship/Chair, CLL). Supported by the NIH/NCI under award number P30 CA016672 and used MDACC Cancer Center Support Grant (CCSG) shared resources.
Combination of ibrutinib and venetoclax is not FDA approved

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal